Fibro Research
The research project, led by Marcia, represents a major milestone in the development of Limbic Reflexology. It is of the utmost importance that we all follow the correct protocols in our contributions to the project. Research findings are subject to intense scrutiny, not only into the concept of the research, but also into the methodology used. No matter how impressive the results, research regularly gets dismissed as invalid, simply on the grounds that the methodology is flawed, and it never sees the light of day, let alone get published.
Follow these guidance notes carefully to ensure that we are all following the strict protocols determined by Marcia’s groundwork, so that we don’t invite criticism of methodology which may undermine the outcomes.
Once we overcome the scrutiny of methodology and consistency, hopefully our work will get the recognition it deserves.
Why Fibromyalgia?
Three reasons
- Fibromyalgia consistently responds very well to Limbic Reflexology. The research aims to demonstrate that.
- Limbic is effective in many problems such as depression and anxiety but there are a multitude of approaches available for these problems in mainstream medicine. However, mainstream medicine offers very little to help those living with fibromyalgia. Any effective affordable treatment is likely to attract attention and potential adoption.
- Recently, Marcia undertook a Master of Research degree at Glasgow Caledonian University. Her final research project was a qualitative study on a participant’s experiences of Limbic Reflexology for Fibromyalgia. Her intention is to have this paper published in a journal.
we are now taking it to the next stage, looking at the efficacy of Limbic Reflexology for Fibromyalgia by doing a mixed method research study both quantitative, using the Revised Fibromyalgia Impact Questionnaire (FIQR) as the outcome measure, and qualitative using qualitative measuring tools.
Over the years, when I worked in mental health in the NHS, I encountered many who were referred for depression, or anxiety, or stress related to fibromyalgia. We could offer help in managing depression etc but this was of limited benefit as frustratingly, we could do little to improve their experience of chronic pain and their dysfunctional lifestyle. Pain clinics offered a lifetime of barely effective medication which often further burdened them with unwanted side effects and including weight gain. This sense failure stayed with me.
Aiming high
One of my first ‘Limbic’ clients was someone living with fibromyalgia. Her dramatic response prompted me to seek out more clients with the syndrome. I did so by enlisting the help of volunteers in the Wakefield Fibromyalgia Support Group, and over the summer of 2012, it became apparent that Limbic was very well suited for helping this client group.
When I discovered that Limbic Reflexology gave those living with fibromyalgia the means to taking back control of their lives, free from the debilitating chronic pain, I set out on a ridiculously ambitious mission to get Limbic adopted by the NHS as the recommended treatment for the management of fibromyalgia. Why aim low?
I am well aware of the many hurdles that lie in the way of my aspiration. Many of those hurdles involve substantial institutional roadblocks. Without credible research, there is no way around them. This research is a major step on the long road to realising the ambition.
The Protocols and Processes
Anonymous Data
To protect the anonymity of submitted data, It’s important that all documents are identified with a unique code. Both the Therapist and participant will be identified only by code.
You will be assigned a Therapist ID. This will not be known to Marcia.
You, as Therapist, will determine a code with which to identify the participant. This could be their initials and a number. Rachel Brown would be RB01. The number is simply to differentiate them from another participant who may be called Richard Baker.
So, in the example above with a Therapist who has an ID of JJ2, the code on all documents would be JJ2RB01
The only document that will have the participant’s personal details is the consent form and the record of attendance form which you will upload separately to Hamish and will not be part of the data submission.
Documents and files
All the documents required for research are found in the Downloads page. Access this using the Downloads sub-menu item under Therapists item
Participant information sheet
This should be given to the participant when you tell them about the research project. Give them time to digest it and answer any queries they may have before asking them to sign the consent form. The consent will thus be informed consent. Make it clear that if they are uncomfortable about their data being submitted, this is quite acceptable and that it will not effect their treatment in any way.
Consent Form
If the participant has understood about the research and they consent to the anonymous FIQR data information being be used for the research, then first enter their code on the form, and then ask them to complete the form, entering their name, signature and date on the consent form. This will not be part of the dat submission. Along with the Therapist Declaration form, this is uploaded separately to Hamish.
Therapist declaration document
You will submit a declaration that the participant has given their consent and has completed a consent form.
Outcome measure documents
Initially, Outcome measures are completed by the participant weekly for eight weeks prior to commencing treatments. So, you will need to download eight of each outcome measure and provide these to the participant with instructions to complete one set each week before commencing treatments. You will need to make arrangements with the participant to return these to you on a regular basis.
Then measures are completed before each treatment and for a final time, one week following treatment twelve.
FIQR
The FIQR is the focus of the research. It is used in all serious Fibromyalgia research worldwide and by employing this measure in our research, the benefits of Limbic can be directly compared to other interventions.
The FIQR will be completed by the participant weekly during the control period, before each treatment and a final time one week following treatment twelve.
The first eight FIQR gives a baseline and each subsequent one measures the effect of the previous treatment. Following the twelfth treatment you need to give the participant an FIQR form and ask them to complete it seven days after the treatment, and then return it to you. That way, the effects of treatment twelve is measured.
Detailed instructions in using the FIQR is found in the ‘completing the FIQR’ section below.
Original FIQR forms need to be scanned. There will be 18 scanned sides. This step can lead to confusion if care is not taken. When scanned files are submitted, it is not always certain which reverse side belongs to which front sheet.. For this reason, check that the participant code and date appears both sides before scanning.
PHQ9/GAD7
The PHQ9, (psychiatric health questionnaire, 9 questions) measures depression and the GAD7 (generalised anxiety disorder, 7 questions) measures levels of anxiety.
Spreadsheet
This is a digital file. Again make sure it is clearly identified with the participant code.
The spreadsheet gives a summary of all the FIQR scores. For each completed FIQR, carefully transcribe all the data from your FIQR forms but not the subtotals or final score . The subtotals and final scores fields will be calculated automatically.
You will need to return the spreadsheet and the scans of the FIQR forms to Marcia. This ensures integrity of data input and by also sending the FIQR forms she can check how the participant answered the questions (raw data).
Completing the FIQR
NB The participant’s personal details must not appear on the FIQR or the spreadsheet. If the participant can be identified on either of these two documents, the submission will be invalid. The only time the participant is identifiable is on the consent form and Declaration form
NB. When scanning the FIQR, enter code on both sides of the FIQR.
So, each time, before handing the FIQR to your participantt, enter their unique code where it says ‘Last Name’ and enter either Male or Female in the First Name field and enter Age of participant in Age field
For some reason, there is no date field on the form. So we have to write this in top right corner. I also enter the treatment number after the date. Write participant code and date on both sides of the form. The reason why we need to identify both sides is that, if you scan each side separately, we need to know which two belong to each other.
Initially, The FIQR is completed by your participant weekly for eight weeks prior to commencing treatments. Then before each treatment and for a final time, one week following treatment twelve.
Note that there are 11 boxes. The left-hand box is 0, not 1. So, the boxes score 0 – 10. This is the most common error both in completing the form and in scoring the measure.
Ask the participant to score each question by ticking a box on the continuum from left low to right high. Make sure they understand that there are 11 and that the left-hand box is 0, not 1.
Sometimes one or two items of the first domain (i.e. function), cannot be answered because a participant has not performed that activity since the last treatment or is physically unable to perform that activity. Ask them to either rate the difficulty for the last time they performed the activity, or, if they can’t perform an activity at all or, score it 10.
Steps In Scoring The FIQR
There are 3 steps in scoring the FIQR
Step 1.
Sum the scores for each of the three domains (function, overall, and symptoms) and enter the score for each domain in the sub total boxes.
This can be a fiddly operation as you are constantly having to establish the column number (I have a ‘cheat’ card which I made by cutting down a FIQR form to a strip of boxes with the numbers in the boxes)
Step 2. (I do steps 2 and three on a scrap piece of paper)
Divide domain 1 sub total score by three ,
Domain 2 sub total score is unchanged
Divide domain sub total score 3 by two.
So, if, for example, the scores in the three sub total boxes are 60, 9 and 40
Domain one score divided by three = 20
Domain two remains unchanged at = 9
Domain three score divide by two = 20
Step 3.
Add the three amended domain scores to obtain the total FIQR score. Enter this in the FINAL TOTAL box
So, in the example above, the total score would be 20+9+20 = 49
Once you have scored the form, you will carefully copy the scores into the spreadsheet. We discuss the spreadsheet next.
Completing the Spreadsheet
Submitting Data
Now you have completed all your research submission documents and files, its time to check everything before final submission.
These are the only documents to submit for the research.
Scans of the original FIQR for each treatment
Spreadsheet with all the scores of all treatments
Checklist
Only when you are certain that all is good, then its time to submit your data.
Before submitting your data, make sure you have gone through the checklist thoroughly to avoid any errors or omissions.)
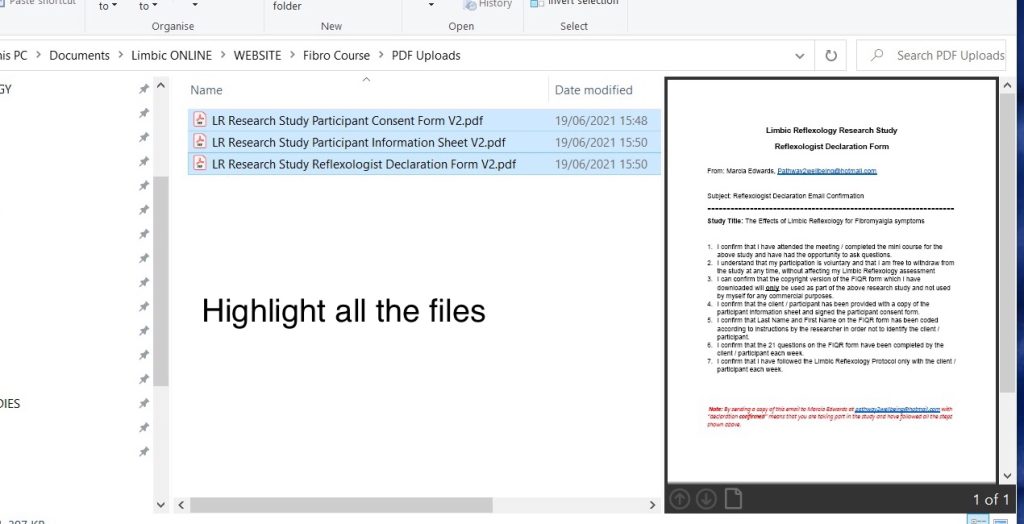
Create zip file
The data files will be uploaded via the upload form. The link to this is found at Therapist > Upload Data in the menu.
There will be a lot of files to upload so to avoid uploading them individually, compress the files into a single zip file.
To do this, on your computer, put all the files for upload in a separate folder. Highlight all the files, then when all the files are highlighted, right click and select save to a compressed zip file. Name the zip file with the participant code. So in the above example, the file name would be JJ2RB01.zip Upload this zip file to Marcia.